Hydrocarbons
Hydrocarbons are simply in the science of chemistry a class of organic matter in which only the atoms of the elements carbon and hydrogen are involved in their molecular structure. Hydrocarbons, like the classification of all organic matter, fall into two groups : aliphatic and aromatic. Alternatively, hydrocarbons can be grouped into two unsaturated and one saturated group.
What is heavy hydrocarbon?
Heavy hydrocarbons One of the unique features of chemistry that has caused us to see its traces everywhere and increase the extent of its borders day by day, is the high fertility and variety of products that come out of it. For example, the components of a simple compound may have many isotopes (the difference between similar atoms in the number of neutrons), which is why this seemingly ordinary compound has different states depending on the atom in which it exists. Situations that sometimes make a big difference.
For example, it may not be easy for some to believe that isotopically we can have 18 different models of a water molecule.
In this combination, having 3 hydrogen isotopes (hydrogen, deuterium and tritium) and 3 oxygen isotopes (represented by numbers 16, 17 and 18) as well as 3 different permutations, there are 2 × 3 × 3 possible states that cause This water molecule can be formed in different ways that heavy water, whose name is often heard, is produced from the combination of heavier isotopes in the same way.
Heavy hydrocarbon analysis
Kian Group International Company is the largest supplier and exporter of petroleum products in Turkey, which with the cooperation of its representative in Iran, Kian Petroleum Company, is able to supply various oil, mineral and steel products directly from the doors of Iranian factories.
Carbon is the most amazing element known
Understanding the importance of individual elements and the extent to which even one or more electrons affect them, carbon is one of the most amazing elements known to man. This element, which has an atomic number of six on the right side of the periodic table of elements and the non-metal part, has four empty orbitals in its last layer, although it is not as reactive as alkali metals, but it is also able to lose electrons in the last layer. It becomes a positive ion and can also be converted to a negative ion by acquiring the same electrons.
Fractional distillation
In fractional distillation, this substance is heated to a temperature of 400 ° C. After this, this fluid is sent into the high distillation tower and during these interactions, smaller and lighter molecules (with one to four carbon attacks) come to the top of the column and heavier and slower molecules (with five to twenty atoms). Carbon and even more) remain at the bottom.
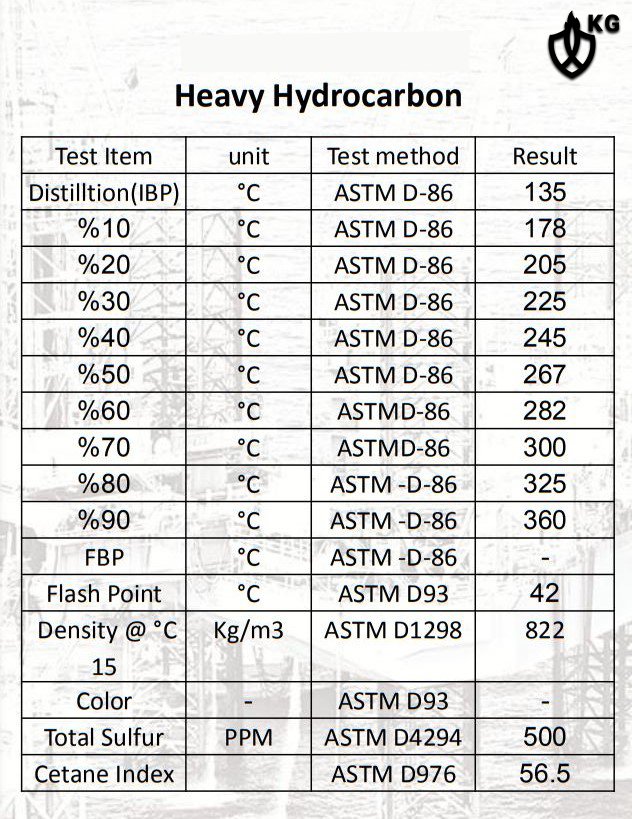
It should be noted that heavy hydrocarbons, which include gasoline, oil and other oils are of the second category. Heavy hydrocarbons have a distillation range of 250 to 400 degrees Celsius and are widely used in internal combustion diesel engines, household burners and other industrial appliances.
This heavy hydrocarbon, also known as diesel or diesel fuel, is in the C14 and C25 ranges. Finally, regarding the functional groups of this product, we can mention paraffinic, naphthenic and aromatic, which have an approximate viscosity of 840 kg / m3.
Intermediate fuel
One of the refinery’s inter-distillate fuels, which has a distillation range of about 150 ° C to 385 ° C and has good performance in domestic and industrial burners of diesel internal combustion engines, is called heavy hydrocarbon.
Hydrocarbons contain various organic compounds that are mainly made up of H and C. In these molecules, the hydrogen-carbon bond is a covalent bond (there is no ionic bond).
Sulfur (S), nitrogen (N) and oxygen (O) atoms are usually substituted for carbon in hydrocarbons. The higher the amount of sulfur in the hydrocarbon compounds, the more it changes the physical behavior and causes a corrosive state. Compounds become heavier and oil refining becomes more difficult. The fewer substituents in the hydrocarbons, the lighter and purer the crude oil.
Production of light hydrocarbons from heavy hydrocarbons
Since most of the country’s extracted oil is heavy and has low economic value, converting heavy hydrocarbon compounds to light is very important. Heavy hydrocarbons have both transportation problems due to high viscosity, high density and و and low economic value due to the difficulty of processing and use in energy production processes. These heavy products are used in low-value sectors such as bitumen and wax production, but will have high value if processed and broken large hydrocarbon chains into lighter products.
Types of oil-producing hydrocarbons
Paraffinic hydrocarbons (alkalis)
Alkalis are the simplest and lightest molecules in the paraffin series, they are simple bonded, they are saturated hydrocarbons, they are chain or linear molecules, they can have a side branch or no branch. Light paraffins are specific to natural gases, and heavier types are found in crude oil. The simplest is methane (CH4).
Petroleum hydrocarbons (cycloalkals or cycloparaffin)
They are the simplest cyclic hydrocarbons, simple bonded, saturated, can have a side branch, tafts are rare in gases but more abundant in crude oils. The purer the crude oil, the higher the amount of naphthenes.
Aromatic hydrocarbons (aromatic)
Cyclic hydrocarbons with simple double bonds have a benzene structural unit and are unsaturated.
Asphalt hydrocarbons
It contains a combination of a large number of aromatic ring molecules, along with the elements O, N, S, which are generally very heavy and unsaturated and do not have a specific general formula. They are mostly of poor quality and concentrated in heavy crude oils or are found in solid oils (natural asphalt).
Introduction of hydrocarbon blending method
This system is for mixing two or more light and heavy hydrocarbons.
In Iran, most manufacturers seek to produce products similar to refinery diesel in this way, which can be sold in the markets of Afghanistan, Pakistan, and Iraq instead of the main diesel of state-owned refineries, because diesel from state-owned refineries is subsidized and exported privately.
Cholera is prohibited at domestic prices, and therefore these factories can find a suitable and similar formulation and lower cost price to sell their product and in a simple and completely legal way to benefit from exports.
It is certain that the hydrocarbons obtained from this process are lower in quality than diesel, but due to the very close similarity of physical properties such as density and flash points, color and transparency, and most importantly, lower cost. For global prices, diesel can easily be sold in neighboring countries such as Afghanistan, Pakistan and Iraq.
Major types of crude oil
Crude oil contains mainly hydrogen and carbon, but in addition it contains small amounts of N, S, O and much smaller amounts of unusual metals, such as vanadium (V) and nickel (N). Although the composition of crude oil is relatively simple, the number of hydrocarbon compounds that may be present in the oil is very large.
There are generally four major groups of compounds in crude oil, including paraffins, naphthenes, aromatics, and resins – asphaltene. Resins and asphaltenes are not pure hydrocarbons and contain elements other than H and C. Paraffins, naphthenes and aromatics are real hydrocarbons. In general, paraffins and naphthenes are saturated hydrocarbons, so that they have enough hydrogen to saturate the electron capacity of carbon atoms. Aromatic hydrocarbons are unsaturated in terms of hydrogen.
The influence of important parameters in determining the quality of hydrocarbon products
- Density / specific gravity: ASTM D1298 / ISIRI197
- Atmospheric Distillation ASTM D86 / ISIRI6261: Atmospheric Distillation
- Vacuum distillation ASTM D1160 / ISIRI890: Vacum Distillation
- Color determination ASTM D1500 / ISIRI20: Color Test
- Drop point ASTM D97 / ISIRI201: Pour Point
- Amount of ash ASTM D482 / ISIRI2940: Ash Content
- Cloud Point ASTM D2500 / ISIRI5438: Cloud Point
- FIash Point
- Closed flash point: ASTM D93 / ISIRI19695
- Open flash point: ASTM D92 / ISIRI198
- Determination of sulfur content ASTM D4294 / ISIRI8402: Sulfur Content
- Determination of water by Karl Fischer method ASTM D6304 / ISIRI18481: Wster Content
- Copper strip corrosion ASTM D130 / ISIRI336: Copper Corrosion
- Mercaptan level: ASTM D3227 / ISIRI9397
- Hydrogen sulfide content: ASTM D 3227 / ISIRI9397
- Gum ASTM D381 / ISIRI18034: Gum Content